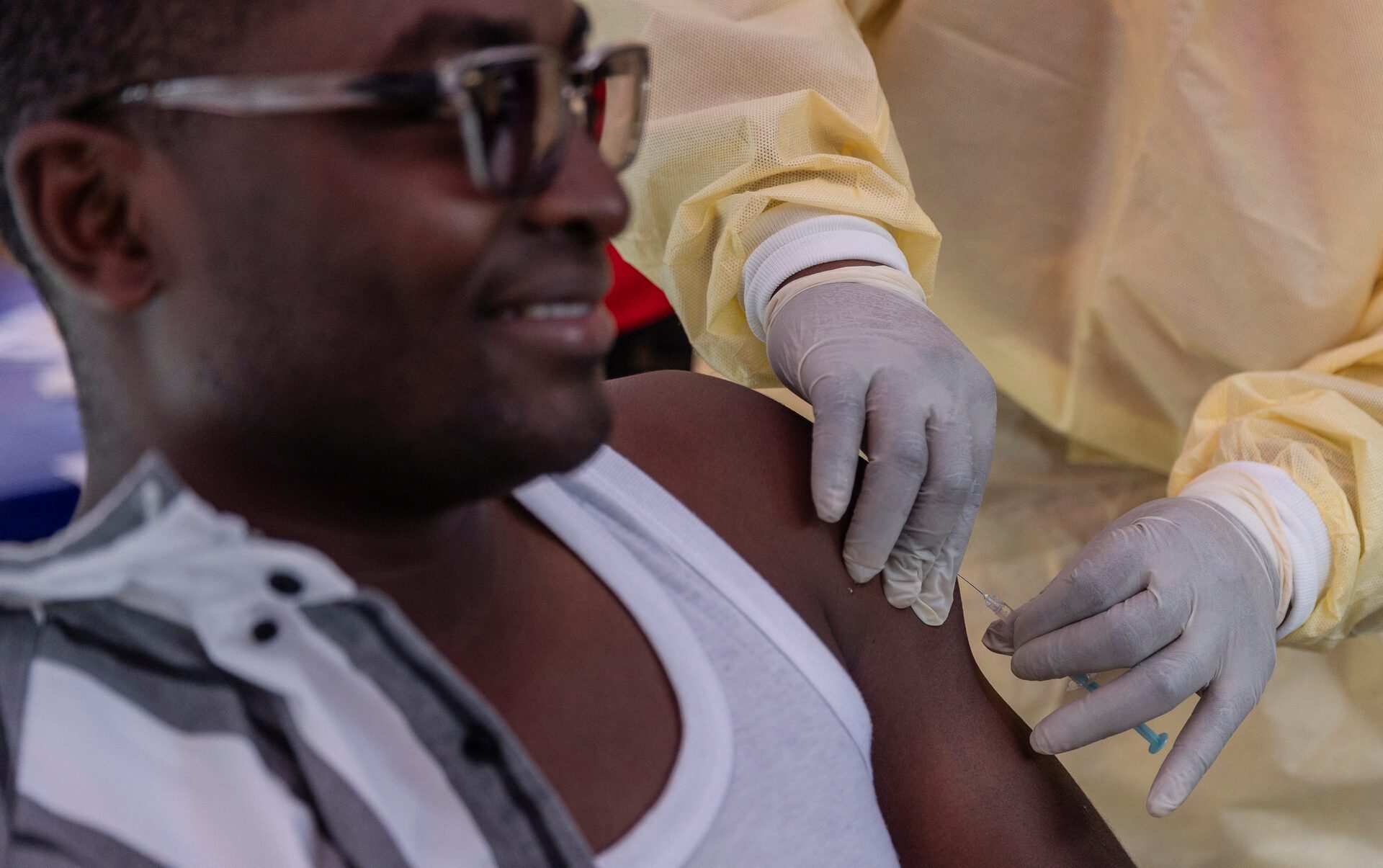
A new donation of mpox vaccines for children in the Democratic Republic of Congo has been delayed due to a longstanding legal issue.
Japan pledged to donate three million doses of the LC16m8 vaccine, the only one approved for use in children. However, a disagreement over liability for potential side effects has held up the shipment.
The vaccine, which was originally developed for smallpox, is crucial in the fight against mpox, particularly for children, who have accounted for the majority of Africa’s more than 1,100 deaths from the disease this year.
While adult vaccinations began in DR Congo’s capital this week, the doses for children remain unavailable. The delay stems from discussions over who would bear responsibility for compensation if side effects occur.
Congo’s health minister Samuel Roger Kamba Mulamba confirmed that the issue has now been resolved, but emphasized that such delays reflect a broader problem within global health responses.
“Both countries must be responsible if any side effects occur,” Mulamba stated, explaining the lengthy negotiations.
Global health experts argue that countries should develop more efficient systems for handling vaccine liability in advance of outbreaks, as delays in response can cost lives.
In contrast, other vaccines for adults, such as the Bavarian Nordic shot, have received full approvals, avoiding similar complications.
Though the Japanese donation for children is crucial, Congo faces challenges in effectively deploying the vaccine, including training health workers in its use. The LC16m8 requires a bifurcated needle, which necessitates additional preparation.
While Japan is working to resolve the remaining issues, the urgent need for vaccines in Congo continues to grow.
