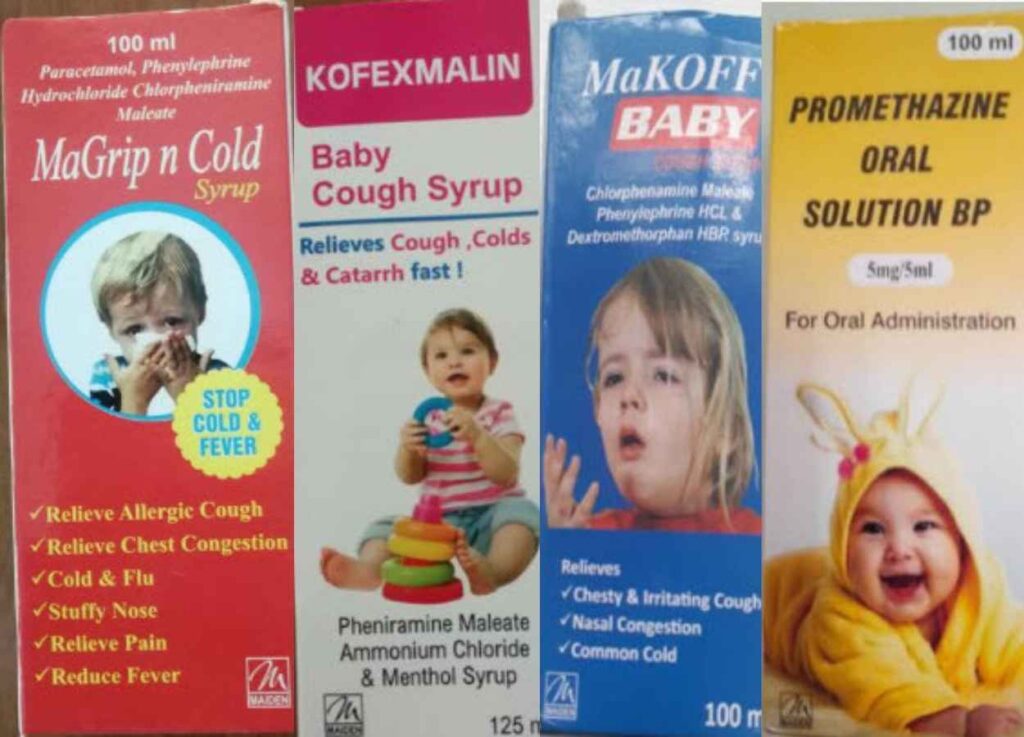
A trial commenced in Banjul, The Gambia, addressing the tragic deaths of young children in 2022 due to a cough syrup manufactured by India-based Maiden Pharmaceuticals.
Following the demise of approximately 70 children under the age of 5, public outrage surged in the West African nation.
Justice Ebrima Jaiteh of the High Court adjourned the proceedings until November 7, noting the absence of three state defendants.
Nineteen plaintiffs, representing the bereaved families, initiated a civil suit in July.
They are demanding acknowledgment from Maiden Pharmaceuticals, Atlantic Pharmaceuticals, the Medical Controls Agency (MCA), the Ministry of Health, and Attorney General Dawda A. Jallow that the contaminated medicines led to the children’s deaths.
Additionally, they seek recognition of the MCA’s negligence in regulating medication safety. Each family is pursuing 15 million Dalasis (approximately $230,000) in damages per child.
Although the defendants were not present during the trial, the health ministry, MCA, and attorney general requested a delay, which the judge dismissed, instructing them to compensate the plaintiffs with 10,000 Dalasis.
The case had already faced delays in July, as the attorney general and health ministry claimed they were not properly served subpoenas.
The article highlights the WHO’s discovery of harmful substances in the cough syrup and the resulting health implications.
It also emphasizes the families’ call for justice and the Gambian government’s proposed actions against the Indian manufacturer following the tragic incident.
